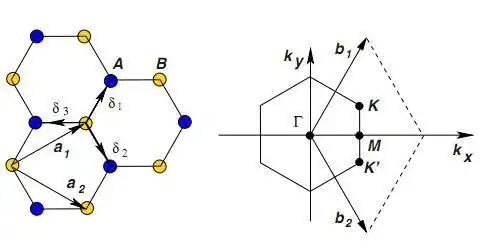
I've been reading about nanotubes lately, and I keep seeing the $ (n,m) $ notation. How does this describe a nanotube's structure? How do I determine which is $n$ and which is $m$ ?
I'm familiar with matrix notation referring to rows and columns, but I couldn't connect it with the nanotube structure which is, albeit predictable, not quite like a row-column grid.