Two dipoles can form a quadrupole, two quadropoles an octopole. The textbook by Griffith then says ' and so on'. So how would a hexadecapole really look like? My impression was that the construction seems to rely on going to higher and higger dimensions.
Asked
Active
Viewed 1,585 times
3
-
4Possible duplicate of Hexadecapole potential using point particles? – Emilio Pisanty Feb 17 '17 at 10:43
1 Answers
3
I think the easiest way to visualise the various multipoles is through their connection with the spherical harmonics. These are probably best known as the functions that give the shape of the atomic orbitals in the hydrogen atom.
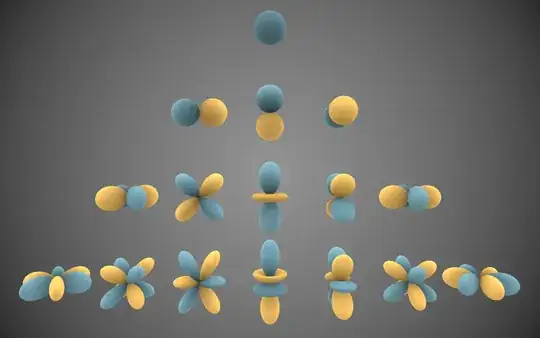
The $p$ orbitals correspond to a dipole, the $d$ orbitals to quadrupole and the $f$ orbitals to an octapole, that is a charge distribution of this shape creates a dipole/quadrupole/octapole. So a charge distribution creating a hexadecapole would look like the $g$ orbitals.
Later:
I've just spotted Hexadecapole potential using point particles?, which gives the same answer I've given so I suppose this question is a duplicate.
John Rennie
- 355,118