In my previous Phys.SE question, I asked for why $ \newcommand{\k}[2]{\langle #1|#2 \rangle} c_1,c_2,c_3,c_4$ in $$\psi_{sp^3}= c_1\psi_{2s}+ c_2\psi_{2p_{x}} + c_3\psi_{2p_y}+ c_4\psi_{2p_{z}}$$ would add to 1 when their squares are summed in order to ascertain whether hybridisation is a quantum superposition.
Now, I know that hybridised orbital is a quantum superposition which implies
$$c_1= \k{\psi_{2s}}{\psi_{sp^3}}, \\ c_2= \k{\psi_{2p_x}}{\psi_{sp^3}},\\ c_3= \k{\psi_{2p_y}}{\psi_{sp^3}},\\ c_4= \k{\psi_{2p_z}}{\psi_{sp^3}};$$ that is, each coefficient is the amplitude for the electron to go from $|\psi_{sp^3}\rangle$ to the respective atomic orbitals.
But, they are more than just amplitudes as said by Peter Atkins in his explanation:
The coefficients in the hybrid have been chosen to give the correct directional properties of the hybrid. The squares of the coefficients give the proportion of each atomic orbital in the hybrid.
Also, the diagram below also shows how for different values of coefficients, the spatial orientation is changed.
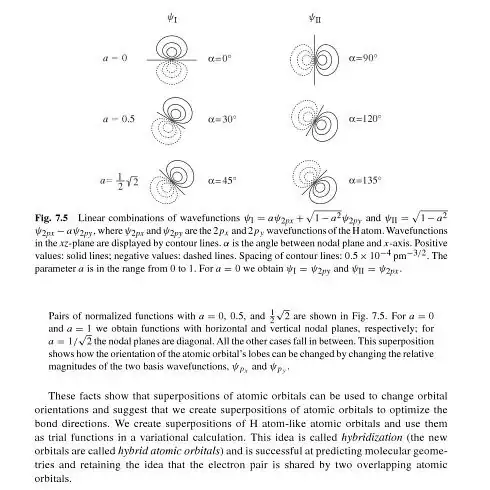
The snapshot above is taken from Principles of Physical Chemistry By Hans Kuhn, Horst-Dieter Försterling, David H. Waldeck
I haven't found any deduction as to how the coefficients determine the spatial orientation. I thought they are just amplitudes to go from the hybridised state to the pure orbital state; how can they direct the spatial orientation? In fact, why would they have to direct the direction of the orbitals. They are probability amplitudes, aren't they? I just want to know how to deduce that these probability amplitudes are actually influencing the direction. How can it be deduced mathematically?

