Sometimes electrons are excited and return to their ground state in one step, while sometimes they take multiple jumps. What determines the path they take?
-
may be it is incoming new photon which collide with the excited atom that determines or also the the excitation star of atom. – Anubhav Goel Oct 31 '15 at 04:14
-
That is just a matter of chance. No particular path is preferred over the other, but someone more experienced than me will be able to elaborate. – Tamoghna Chowdhury Oct 31 '15 at 05:02
-
Electrons don't take any paths, at all. Atoms change state trough the emission (better creation in a field theory terminology) of one or multiple photons. Which process happens depends on the symmetries of the initial and final state (and intermediate states if they exist) and the form of the interaction Hamiltonian. – CuriousOne Oct 31 '15 at 05:37
-
2@CuriousOne Yet if I hold a photon detector next to an excited atom, some times I get three photons of one energy unit each, and some times I get one photon of one energy unit and one photon of two energy units. – DanielSank Oct 31 '15 at 05:46
-
@DanielSank: What does a multi-photon process have to do with paths???? – CuriousOne Oct 31 '15 at 05:58
-
1@CuriousOne well, in this case, the fact that the question suggests that the word "path" should be interpreted as "set of states occupied by an electron as it goes from an excited state to the ground state". Note the use of the word "step" and expression "multiple jumps" in the question text. Perhaps you only read the title? – DanielSank Oct 31 '15 at 06:12
-
@DanielSank: So it's an English question, then? You are right, I am not an English major. :-) – CuriousOne Oct 31 '15 at 06:19
-
@CuriousOne I estimate roughly 75% of confusion in life comes from imperfect communication. Physics is no exception. We shouldn't laugh it off. Getting the terms right matters a lot. – DanielSank Oct 31 '15 at 06:23
-
@DanielSank: You don't have to tell me... just look at some of the other questions like the one about the "purity of energy". Physics starts with such simple definitions and almost nobody takes the time to read them... sigh. – CuriousOne Oct 31 '15 at 07:43
4 Answers
'Path' is perhaps a misleading word to use here. Path typically means a physical path through space - a trajectory. You can use path in the context of a path through a sequence of energy states, talking about which energy states an electron is in in which order, but you have to establish the context to use the word like that before hand.
Back to the actual question: what determines how many photons are emitted? It is random.
Consider a simple system where an electron can have three energy states, the lowest being $E_{0}$, then $E_{1}$ and $E_{2}$. An electron in state $E_{2}$ can either transition directly to $E_{0}$ emitting a photon where $h\nu=E_{2}-E_{0}$, or it can transition to $E_{1}$ and then $E_{0}$, emitting two photons (of energy $E_{2}-E_{1}$ and $E_{1}-E_{0}$).
It is possible to calculate transition probabilities per unit time for each possible transition. So in any given time period there will be a certain probabilty $p_{20}$ that the electron drops to state $E_{0}$ and a probability $p_{21}$ that the electron drops to state $E_{1}$. And of course, a probability that it remains in its current state.
Once those probabilities have been calculated, you can figure out how often an electron will return to the ground state via emitting a single photon, vs two photons (in our simple system). But there is nothing deterministic to say which path will be taken (see, now we can use path without anyone arguing about what it means... :) ). It is entirely random. All we can do is figure out the probabilties involved.
- 2,394
It might not take a particular path at all.
Some transitions might be strongly forbidden based on the difference of angular momentum of the states. But you can also have double photon transitions directly between two states, or have multiple transitions that have the final state of one transition be the starting state of the other.
And if you take that last approach, you could have a series of transitions between A and D through state B and another series of transitions between A and D through C and in addition to doing one series or the other series there could be a superposition of both.
In fact, a similar thing happens in photosynthesis where different ways to collect energy from sunlight can constructively interfere and be more efficient together than the sum of the two approaches alone would be.
- 25,523
In general, electronic relaxation of an excited atom is nothing but a quantum mechanical transition from an initial to a final state. Therefore probabilities for different transitions (what you called paths) is determined by the rules of quantum mechanics.
The particular "law" that applies here is called Fermi's Golden Rule. In the related Hyperphysics page is says
In general conceptual terms, a transition rate depends upon the strength of the coupling between the initial and final state of a system and upon the number of ways the transition can happen (i.e., the density of the final states). In many physical situations the transition probability is of the form

- 2,352
OK my previous answer was not cited correctly. I am trying to give you a good guess on what is going on when an electron returns to ground state. This is cited from another site, as I said I would just like to help you with clearing up this process with electron excitation.
You are asking specifically what determines whether they go 'straight' to ground state or step-by-step.
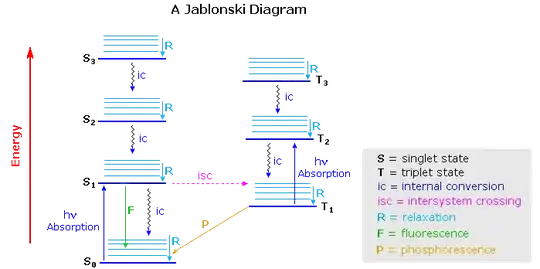
"A molecule that is excited can return to the ground state by several combinations of mechanical steps (see the picture). The deactivation process of fluorescence and phosphorescence involve an emission of a photon radiation. The wiggly arrows are deactivation processes without the use of radiation. The favored deactivation process is the route that is most rapid and spends less time in the excited state.If the rate constant for fluorescence is more favorable in the radiationless path, the fluorescence will be less intense or absent."
Here are the different types of deactivation (how the electron goes back to ground state on the picture):
This is here a citation from the original page just for your info:
"Vibrational Relaxation: A molecule maybe to promoted to several vibrational levels during the electronic excitation process.Collision of molecules with the excited species and solvent leads to rapid energy transfer and a slight increase in temperature of the solvent. Internal Conversion: Internal conversion is an intermolecular process of molecule that passes to a lower electronic state without the emission of radiation. External Conversion: Deactivation of the excited electronic state may also involve the interaction and energy transfer between the excited state and the solvent or solute in a process called external conversion. Intersystem Crossing: Intersystem crossing is a process where there is a crossover between electronic states of different multiplicity the singlet state to a triplet state (S1 to T1). Phosphorescence: Deactivation of the electronic excited state is also involved in phosphorescence. After the molecule transitions through intersystem crossing to the triplet state, further deactivation occurs through internal or external fluorescence or phosphorescence. A triplet-to-singlet transition is more probable than a singlet-to-singlet internal crossing. In phosphorescence, the excited state lifetime is inversely proportional to the probability that the molecule will transition back to the ground state. Since the lifetime of the molecule in the triplet state is large (10-4 to 10 second or more), transition is less probable which suggest that it will persist for some time even after irradiation has stopped. Since the external and internal conversion compete so effectively with phosphorescence, the molecule has to be observed at lower temperature in highly viscous media to protect the triplet state."
- 28,452
-
I talked to ACuriousMind, he told me it is technically OK. I really want to just help these people with the information, since I see they did not get a full answer. – Árpád Szendrei Dec 08 '16 at 21:29