Okay, so everywhere I've read, I hear the main difference is the requirement of a medium. But for example, if you take the case of heat 'radiating' from a red-hot iron, isn't that actually convection and not radiation? I mean, isn't the temperature difference between the surrounding air and the iron causing the air to gain heat?
-
1Good answers. Radiative heat transfer uses light, basically. It's how the sun heats us. Then there's conduction - put something hot in contact with something less hot, and heat flow from one to the other. Convection is just a case of conduction, where the one of the things is a fluid that can expand and then rise by gravity, so it carries heat away. Think hot-air balloon, or thunderstorm. – Mike Dunlavey Mar 30 '12 at 21:19
-
1And note: normally, all 3 forms of heat transfer are occurring at the same time. – David White Sep 29 '19 at 02:36
-
Related:https://physics.stackexchange.com/q/24489/226902, https://physics.stackexchange.com/q/62423/226902 and links therein. – Quillo Mar 28 '23 at 10:41
4 Answers
To pretty much everything you stated in your question, "no".
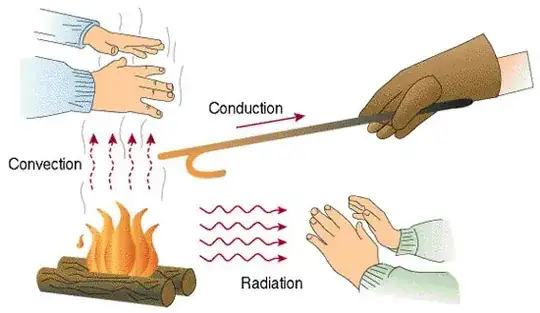
That convection requires a medium is not the main difference, it is simply the most obvious aspect of what is a fundamentally different mechanism for transferring energy. Convection is the transfer of energy by movement of a medium, whereas radiation is the transfer of energy by, well, thermal radiation. Conduction also requires a medium, but, again, it is a fundamentally different mechanism than either convection or radiation; in this case it is the transfer of energy through a medium.
Unfortunately, analogies are hard but if you can visualize the particles involved, it would help. Picture the red hot iron you mentioned. On a molecular level, the material is emitting lots and lots of photons (hence why it is glowing red). The creation of these photons takes energy; energy from the heat of the iron. These photons leave the iron, pass through the environment, and eventually collide with some other object where they are absorbed and deposit their energy. This is radiative heat transfer. If that energy is deposited on your retina or a CCD (like in a digital camera), an image forms over time. This is how infrared goggles work and they would work equally well in high vacuum as here on earth.
In conduction, the next simplest example, there is no generation of photons (physics nerds forgive me for the sake of simplicity). The individual atoms in the object are vibrating with heat energy. As each atom gains energy from its more energetic neighbors, so it gives up energy to its less energetic ones. Over time, the heat "travels" through the object.
In convection, the molecules of gas near the object gain energy, like in the conduction case, but those same molecules that gained energy then travel through the environment to some other location where they then give off their heat energy.
In summary:
- radiation = generated and absorbed photons
- conduction = molecules exciting their neighbors successively
- convection = molecules heated like in conduction, but then move to another location
- 1,335
- 3
- 13
- 23
- 4,963
-
I think radiation should include more than just photons and a general definition of it should reflect this. I would say radiation is particle transport from one place to another that is not part of the bulk medium. – Alan Rominger Mar 30 '12 at 19:22
-
This is true, but how then would you differentiate between convection and radiation? It is the production and re absorption of particles that is the hallmark and, while not all of that radiation is via photons, the vast majority of it is. – AdamRedwine Mar 31 '12 at 18:26
-
I considered saying radiation doesn't move by diffusion but, no, that's wrong too. Photons are probably the "majority" (not sure about definition here) of radiation because they can be super high entropy, and blackbody radiation allows low energy photons to be the end-state of most matter-energy. Radiation also can't be defined by being "temporary". This is why I used "not part of the bulk medium", which is pretty dry but the most correct wording I can come up with. Does conduction happen is gas btw? Maybe it's not even "vibrational". Frustrating, but very good question. – Alan Rominger Apr 01 '12 at 17:20
-
Yes, conduction happens in gas, though to a lesser extent than in solids. Vibrational is not really accurate wording either, but it is about as good as you can get at this level of explanation. I took thermo in physics undergrad and several times in engineering grad school so my wording tends to skew toward the intuitive engineering side of things. – AdamRedwine Apr 02 '12 at 12:25
I will try to explain in simple words.
Every body which has a temperature above 0 Kelvin gives out (ie. radiates) some heat in the form of waves. (So, even we radiate!) Of course, the amount of this radiation depends on the temperature, so the more the temperature of the body, the more heat it gives out. Now, since this heat energy travels in the form of waves, it does not necessarily require any medium to travel. So, it can travel in any/no medium.

I will try explaining convection using a simple example.
Consider a beaker of water being heated from the bottom. The water in the lower region gets heated up, becomes lighter in weight, and hence comes to the top. Now the (relatively) cooler region of water on the top comes down and begins to heat up. Now, again it gets heated up, and moves up when it becomes lighter than the (previously heated) water on the top, but this time getting more heated that the water on the top. This process continues, and eventually every molecule of water gets heated up.
As you can see, in this process, the motion of the particles lead to the heating of the whole body (water, in this case) - the warmer ones moved away from the source of heat to let the cooler ones collect the heat.

So, its clear that convection requires a medium (specifically, a non-solid medium). Unlike radiation, if there is no medium near the source, it cannot loose its heat simply using convection. (It can of course loose it via radiation.)
Lets comes to your heating of iron rod case.

What you said is partly correct, that convection is one of the modes of heat transfer here. But, so is radiation. Remember, that the iron rod is too hot as compared to the surrounding temperature and so it will radiate a lot of heat. In fact, the heat is so much that the rod glows bright red. (If you know a little about the EM spectrum, you would know that when the emission from a body also includes the visible spectrum, we can actually see (a part of) the emission spectrum.)
In reality, all 3 modes of heat transfer occur simultaneously.
(Even in the above beaker example, the water molecules, (along with convection) give out heat in the form of radiation as well, as they have a non-zero temperature. They also transfer heat by collisions to other water molecules, which is known as conduction. However, in that example, convection was the most dominant mode of heat transfer.)
If you wish to know the exact difference between these modes of transfer, you would perhaps need to take up intermediate-level engineering course.
- 241
-
In your graphical examples, convection is hopelessly intermingled with the side effects of the fluid having weight, or otherwise interacting with a potential field. The heat rising and convective cells are not key properties of convection itself. Once the medium is not in a potential field (e.g. is weightless), they vanish, yet convection itself certainly doesn't! The word convection is, unfortunately, used to describe both the heat transfer mechanism and the motion of the fluid in a potential field in a temperature gradient. – Kuba hasn't forgotten Monica Nov 06 '16 at 02:04
No. Light (as you can see --- it's red hot! --- and infrared light which you cannot see) leaves the metal surface and reaches your skin/thermometer directly, and would do so without air.
- 8,669
The answers already given explain the differences between the processes by which heat is transferred from one body to another but there are also differences in their relative importance in particular situations.
A central heating radiator does radiate heat but convection is a more important process once the air is heated and before that conduction through the metal of which the radiator is made is the dominant process.
At very low temperatures radiation is the dominant process because most substances are solids and are very poor conductors of heat.
At very high temperatures radiation is the dominant process
So temperature is a factor which can determine which process is the most important one.
Your red hot iron is a good example in that if you put your hand close to it you can "feel" the radiation and because the temperature of the iron is relatively high there will be a lot of radiation (mostly infra red) emitted from it but also there will be a loss due to convection.
To identify and then quantify which process is the most important is often very difficult.
After a light bulb has been on for a period of time the glass envelope gets hot but the processes by which it gets hot are quite complex.
Try the following.
Choose an accessible light bulb which has not been on for some time.
Hold your hands around the light bulb without actually touching it.
Switch on the bulb and then after a few seconds switch it off.
You will have felt the sensation of heat due to the radiation emitted by the heated filament but if you now touch the glass envelope it will still be cold.
So radiation is very important particularly as that is in part light but the majority of the energy transfer from the filament is in the infra red.
- 95,680