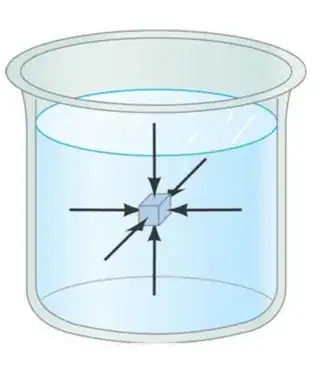
Consider an imaginary cube of liquid as shown in the figure.
Why should all the forces on the cube be same? The cube can be stationary even if the forces on parallel, opposite vertical sides are equal and opposite in direction. The cube can be arbitrarily small or large.
Edit $-$
Based on the answer by Steeven, I just want to consider a similar hypothetical experiment of that sort. Imagine a container with rigid, smooth balls. If I apply pressure $P$ on all the sides of this container, will I get this much amount of increased pressure on the upper and lower sides? I think that more the spheres are small and more in number, the more pronounced the above-mentioned effect will be seen.(This is my guess)