In addition to Pieter's answer which claims that during a phase transition adding more heat does not contribute to a change in kinetic energy, I add that in some phase transitions, adding more heat converts a liquid to a solid, thereby lowering the kinetic energy of the molecules.
I am not sure I agree with Pieter's answer, because I'd argue that even though the temperature stays the same during a phase transition, if liquid is converted to gas or solid is converted to either liquid or gas, the kinetic energy of the molecules do increase.
Edit: As requested by Pieter, a concrete example of a liquid material that becomes a solid when heat is added is helium 3. See the corresponding phase diagram: 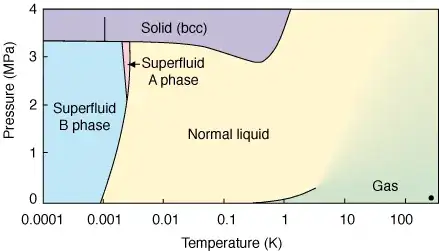
Note that it isn't the only substance having this property. I do not remember the exact name of such substances, but I do remember a good thermoelectric one having that property too.
