What is a wave?
A physical observation of changes in space , as water waves, sound waves ....
What is a wave function?
A solution of differential equations called wave equations.
The wave equation is a second-order linear partial differential equation for the description of waves—as they occur in classical physics—such as mechanical waves (e.g. water waves, sound waves and seismic waves) or light waves.
Depending on the observations , the solutions can be used to model it mathematically, and those solutions can predict the behavior of the wave in time.
In order for the mathematical solutions to be descriptive and predictive extra axiom type postulates have to be imposed, to pick up those solutions that will be useful in modelling and predicting physics data.
We then come to the wave differential equations used for modeling behavior of matter at the quantum mechanical level. The extra axioms and assumptions in order to model data are seen here.
The simple particle in a box is a particular potential used in the Schrodinger wave equation.
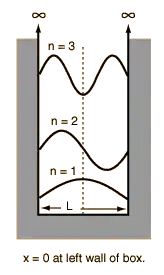
Assume the potential U(x) in the time-independent Schrodinger equation to be zero inside a one-dimensional box of length L and infinite outside the box. For a particle inside the box a free particle wavefunction is appropriate, but since the probability of finding the particle outside the box is zero, the wavefunction must go to zero at the walls. This constrains the form of the solution to
Due to the boundary conditions the solutions are constrained mathematically for this simple case, because the boundary conditions are clear. (The infinite potential outside the wall, the zero inside).
In the general differential wave equations used for modeling quantum mechanical states, (Schrodinger,, Dirac,Klein Gordon, quantized Maxwell) if no potential exists, the solutions are plane waves .
Is there some sort of one to one mapping between potential energies and wave functions?
Take the Schrodinger solutions for the hydrogen atom . There are constraints on the wave equation that pick them up from the possible mathematical solutions:
These constraints are applied to the boundary conditions on the solutions, and in the process help determine the energy eigenvalues.
This should answer your question, because if these constraints are not imposed in picking up useful for modeling wavefunctions a large number of wavefunctions exist. It is not possible to pick the ones contributing to the hydrogen solutions ones by just looking at all the solutions.
I will quote from the link Zachos gave:
In general, the answer is no. This type of inverse problem is sometimes referred to as: "Can one hear the shape of a drum"
This should apply to your one to one mapping too.
