Homogeneity = translational invariance
A material is homogeneous with respect to the property $f$ (for example density) if
$$f(\mathbf r) = f (\mathbf r + \mathbf r')$$
i.e. property $f$ does not depend on the spatial position. If you measure property $f$ at point $\mathbf r$ or $\mathbf r+\mathbf r'$, you will find the same result.
Examples: most materials are homogeneous at a large enough scale, but they can reveal inhomogeneities if we look close enough. See the section about scale.
Isotropy = rotational invariance
A material is isotropic with respect to the property $f$ if
$$f(\mathbf r) = f (|\mathbf r|)$$
i.e. property $f$ does not depend on the direction of its argument. If you measure property $f$ along any direction in the material, you will find the same result.
Examples: fluids and amorphous solids are isotropic. Most crystals (with a few exceptions like the cubic crystal system) are not isotropic.
Scale dependence
Notice that both homogeneity and isotropy are scale-dependent quantities: they depend on the spatial scale where we choose to effectuate our measurements.
To give you a specific example, consider steel: steel is an iron-carbon alloy. At a large enough scale (let's say the mm scale), steel is homogeneous. However, if you look at it close enough ($\mu$m scale), this is what you see (source):

Definitely not homogeneous. Another example is granite:

Other examples of materials which are homogenous/isotropic on large scales but inhomogeneous/anisotropic on smaller scales, apart from alloys, are polycrystalline materials.
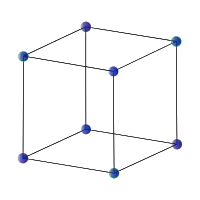
Also a normal simple cubic crystal (figure below), which is isotropic on large scales, is anisotropic on small scales. To see this, just think about standing in the center of the cube: how many atoms will you encounter if you move towards one of the faces? And how many if you move along one of the diagonals? The answer is different.
To conclude, I will just remark that homogeneity and isotropy are independent from each other. Below you can see an homogeneous but not isotropic pattern on the left and an isotropic but not homogeneous pattern on the right (source).

Edit: I should have specified this, but a material can be isotropic with respect to a point. The figure on the right is isotropic with respect to its center, however it is clearly not isotropic with respect to all points. If it was isotropic with respect to more than 1 point, it would also be homogeneous.



